Woman implanted with 3D-printed ear made from her own cells
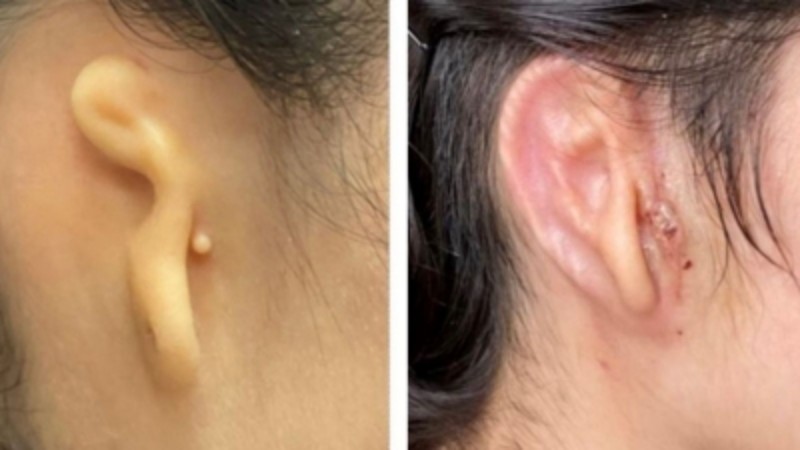
Woman implanted with 3D-printed ear made from her own cells (Photo: Microtia-Congenital Ear Institute and 3DBio Therapeutics Via IANS)
Woman implanted with 3D-printed ear made from her own cells (Photo: Microtia-Congenital Ear Institute and 3DBio Therapeutics Via IANS)
San Francisco | A 20-year-old woman has become the first in the world to get a human ear implant with a 3D-printed technology that uses her own cells.
The AuriNovo implant, developed by 3DBio Therapeutics (3DBio) — a clinical-stage regenerative medicine company — is a patient-specific, living tissue implant created using 3D-bioprinting technology.
The implant is specifically for patients with microtia, a rare congenital deformity where one or both outer ears are absent or underdeveloped.
In the first clinical trial of the technology, doctors reconstructed the ear of the woman using the patient’s own cartilage cells. The construct was then printed in a size and shape matching the patient’s opposite ear for implantation.
It is a “groundbreaking reconstructive procedure”, 3DBio said in a statement.
The current surgical methods for ear reconstruction requires the harvesting of rib cartilage or the use of porous polyethylene (PPE) implants.
But, “the AuriNovo implant requires a less invasive surgical procedure than the use of rib cartilage for reconstruction,” said Dr. Arturo Bonilla, who performed the transformational implant procedure.
“We also expect it to result in a more flexible ear than reconstruction with a PPE implant. The AuriNovo living tissue implant is designed to provide a better solution for patients born with microtia by transforming their appearance and building their confidence and self-esteem,” added Bonilla, who is also the founder and director of the Microtia-Congenital Ear Deformity Institute in San Antonio, Texas.
The Phase 1/2a clinical trial, which aims to involve 11 people, will be conducted at Los Angeles, California and San Antonio, Texas
Pending the outcome of this trial, researchers will conduct a second and larger trial before seeking US Food and Drug Administration (FDA) approval of AuriNovo, which has also been granted orphan drug and rare paediatric disease designations by the regulatory body, meaning that it will receive a priority review when ready.
“This is a truly historic moment for patients with microtia, and more broadly, for the regenerative medicine field as we are beginning to demonstrate the real-world application of next-generation tissue engineering technology,” said Daniel Cohen, 3DBio Chief Executive Officer and Co-founder, in a statement.
Cohen stated that the success of the technology could demonstrate its potential to provide living tissue implants in other therapeutic areas in the future.
“Our initial indications focus on cartilage in the reconstructive and orthopaedic fields including treating complex nasal defects and spinal degeneration,” Cohen said.
“We look forward to leveraging our platform to solve other high impact, unmet medical needs like lumpectomy reconstruction and eventually expand to organs,” he added.
IANS